About Alnylam
Founded in 2002, Alnylam was built upon a bold vision of turning scientific possibility into reality and is now advancing a new class of innovative medicines to address the needs of patients who have limited or inadequate treatment options. Our robust pipeline of investigational RNAi therapeutics is currently focused on several disease areas including transthyretin amyloidosis, rare diseases, cardiovascular diseases, metabolic diseases, and neurological diseases.

Resources
Copyright © 2025 Alnylam Pharmaceuticals, Inc. -- All materials and resources provided on this site by Alnylam Pharmaceuticals, Inc. (the “Media Kit”) are protected by copyright and other intellectual property laws. The Media Kit provided on this website is intended for informational purposes only. Unauthorized use, reproduction, or distribution of any of these materials for commercial purposes is strictly prohibited and may result in legal consequences. By downloading any content from this Media Kit, you agree to use it solely for non-commercial purposes and in accordance with the terms outlined herein.
To request permission to use Media Kit content for any purposes other than the ones expressly authorized above, please contact media@alnylam.com.
Corporate Backgrounder
A synopsis of our RNAi therapeutics, experience, and active pipeline.
- Download435.72 KB

RNAi Fact Sheet
Learn about the history of RNAi and what it means for the advancement of human therapeutics.
- Download447.55 KB

Corporate Responsibility Report
Our Corporate Responsibility Report details our approach to corporate responsibility and current and planned efforts in our five key areas of focus.
- DOWNLOAD 2024 REPORT46.73 MB
- DOWNLOAD 2023 REPORT7.04 MB
- DOWNLOAD 2022 REPORT12.72 MB

Patient Access Philosophy
Our annual readout on progress against the pillars of our Patient Access Philosophy.
- Download 2024 Report244.5 KB
- Download 2023 Report185.15 KB
- Download 2022 Report165.5 KB


Alnylam Rare Disease Trend Report
Our Rare Disease Trend Reports are based on survey responses from >30 U.S. payer and plan decision-makers and provides a unique window into the minds of insurers about orphan drug access.
- DOWNLOAD 2024 REPORT21.6 MB
- DOWNLOAD 2023 REPORT15.29 MB
- DOWNLOAD 2022 REPORT8.57 MB

RNAi Activity Booklet for Kids
Learn about the basic science of RNAi through fun activities such word search, connect the dots, matching, and coloring. This activity booklet is intended for families and children aged 7-14.
- Download18.87 MB

Management Board
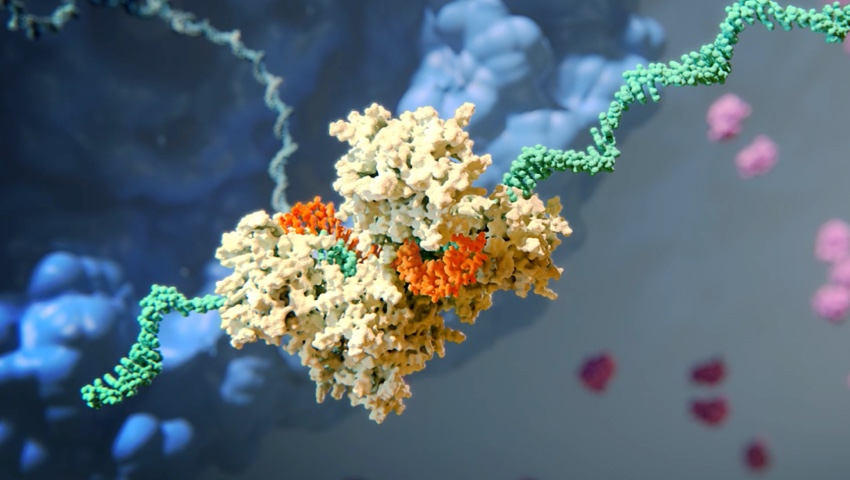
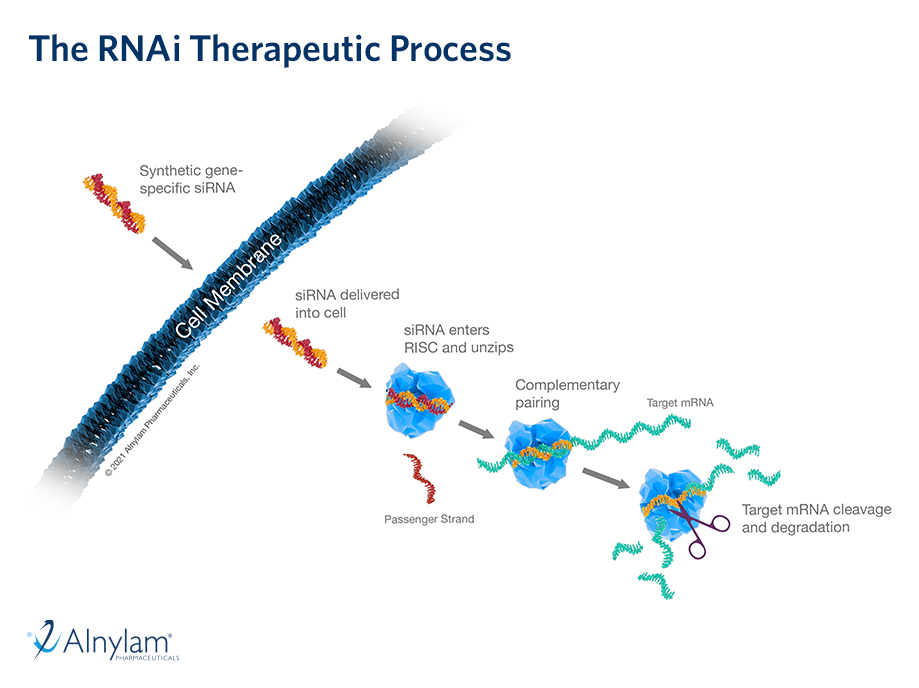
The Science of RNAi
RNA interference (RNAi) is a breakthrough in understanding how genes are regulated in cells. It also represents a completely new approach to drug discovery and development.
Read More ›Download(Format: Jpeg 173 KB)
Disease Information
About Acute Hepatic Porphyria
Acute hepatic porphyria (AHP) refers to a family of rare, genetic diseases characterized by potentially life-threatening attacks and, for some patients, chronic debilitating symptoms that negatively impact daily functioning and quality of life. AHP comprises 4 subtypes, each resulting from a genetic defect leading to deficiency in one of the enzymes of the heme biosynthesis pathway in the liver: acute intermittent porphyria, hereditary coproporphyria, variegate porphyria, and ALAD-deficiency porphyria.
About ATTR Amyloidosis
Transthyretin-mediated (ATTR) amyloidosis is an underdiagnosed, rapidly progressive, debilitating and fatal disease caused by misfolded transthyretin (TTR) proteins. The misfolded TTR proteins collect as amyloid deposits throughout the body, including the nerves, heart and digestive system, resulting in progressive organ damage. There are two different types of ATTR amyloidosis – hereditary ATTR (hATTR) amyloidosis, which is caused by an inherited variant, or change, in the TTR gene, and wild-type ATTR (wtATTR) amyloidosis, which occurs without a TTR gene variant.
About Primary Hyperoxaluria Type 1
Primary hyperoxaluria (PH) constitutes a group of rare inherited disorders of the liver characterized by the overproduction of oxalate, an end-product of metabolism. There are 3 types of PH: type 1 (PH1), type 2 (PH2), and type 3 (PH3). PH1 is the most common and severe form, accounting for 70% to 80% of all cases. PH1 affects approximately 4 individuals per million in the United States and Europe, with an estimated 1,300 to 2,100 diagnosed cases. In some regions, such as the Middle East and North Africa, the genetic prevalence of PH1 is higher.
About Hypertension
Hypertension, also known as high blood pressure, is a complex multifactorial disease clinically defined by most major guidelines as a systolic blood pressure (SBP) of above 140 mm Hg or a diastolic blood pressure (DBP) greater than 90 mm Hg, though some guidelines have a lower threshold of a SBP above 130 mm Hg or a DBP greater than 80 mm Hg. More than one billion people worldwide live with hypertension, and fewer than 20 percent of those affected have it under control.
Clinical Trials
Our clinical studies allow us to evaluate whether potential new treatments are safe and effective.
APOLLO and APOLLO-B Phase 3 Studies
APOLLO evaluated the efficacy and safety of patisiran in patients with the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis, and APOLLO-B is evaluating patisiran in patients with the cardiomyopathy of wild-type transthyretin-mediated (wtATTR) amyloidosis or hATTR amyloidosis.
Download Fact Sheet(Format: PDF)

ENVISION Phase 3 Study
The ENVISION Phase 3 study evaluated the efficacy and safety of givosiran in patients with acute hepatic porphyria (AHP).
Download Fact Sheet(Format: PDF)

ILLUMINATE-A, -B, and -C Phase 3 Studies
ILLUMINATE-A randomized, double-blind, placebo-controlled Phase 3 study evaluating safety and efficacy of lumasiran in children (age 6 or older) and adults with PH1. ILLUMINATE-B open-label, multicenter Phase 3 study evaluating safety and efficacy of lumasiran in infants and young children (under the age of 6) with PH1. ILLUMINATE-C open-label, multicenter Phase 3 study evaluating safety and efficacy of lumasiran in PH1 patients of all ages with advanced renal disease, including patients on dialysis.
Download Fact Sheet(Format: PDF)

HELIOS-A and -B Phase 3 Studies
Evaluating the efficacy and safety of vutrisiran in patients with hereditary transthyretin amyloidosis (hATTR amyloidosis) and with transthyretin amyloidosis cardiomyopathy (ATTR amyloidosis with cardiomyopathy).
Download Fact Sheet(Format: PDF)

KARDIA Phase 2 Studies
Evaluating the efficacy and safety of zilebesiran as monotherapy across different doses administered quarterly and biannually in patients with mild-to-moderate hypertension, and when used in combination with conventional antihypertensive medications.
Click here to see all of our clinical studies.


You will be redirected to an external site that may include social media, event registration, or other third-party content related to Alnylam.
Please note that these external sites have their own terms of service and privacy policies, which may differ from those of Alnylam.
Proceed to Site